Where Are the Products in a Chemical Equation Located
The pyruvate oxidation location is the mitochondrial matrix of eukaryotic cells. The equation becomes F e 4 H 2 O F e 3 O 4 H 2.

Chemical Reactions Chemical Reactions Chemical Reactions
Krebs cycle takes place in mitochondria in eukaryotes whereas in prokaryotes it takes place in the protoplasm.

. The is read as reacts with and the arrow means produces. A chemical reaction involve rearrangement reactants to form products. Humans other mammals plants fungi etc are organisms that contain a nucleus nuclear envelope.
Where are reactants and products located in a chemical equation. Reactants are written on the left side of a chemical reaction while products are written on the right hand side of chemical reaction. It is the convention to place the reactants on the left side of a chemical equation and the products on the right side.
Such equations are known as thermochemical equations. Chemical reactions are represented on paper by chemical equations. Give the balanced equation for solid iron III sulfide reacting with gaseous hydrogen chloride to form solid iron III chloride and hydrogen sulfide gas.
Methane oxygen carbon dioxide water The arrow is read as react to yieldor yield alsoproduceor form. The reactants the starting substances are written on the left and the products the substances found in the chemical reaction are written on the right. Thank you for your time.
Question 1 of 26 How many oxygen atoms are located on the reactant side of the. For example hydrogen gas H 2 can react burn with oxygen gas O 2 to form water H 2 0. They are the elements and compounds located on LEFT side of a chemical equation.
At the beginning of the chemical equation o o. In a chemical equation where do the reactants appear-. Every molecule of Fe₂O₃ contains 2 Fe atoms and 3 oxygen atoms.
View the full answer. Where are the products in a chemical equation located. The word equation for the reaction of methane and oxygen is written as follows.
Between the reactants of the chemical equation. In the reaction methane and oxygen are the reactants and carbon dioxide and water are the products. What is located on the right side of a chemical equation.
Product is located on the right side of the equation. The left side of the equation and products are placed on the right. Reactant are located on the left side of the equation and the product is located on the right side of the equation.
The coefficients next to the symbols of entities indicate the number of moles of a substance produced or used in the chemical reaction. A product is a substance that is present at the end of a chemical reaction. The given reaction is 2Al s Fe2O3 s Al2O3 s 2Fe l The substance s to the left of the arrow in a chemical equation are called reactants.
2 Al s Fe₂O₃ s Al₂O₃ s 2 Fe l Oxygen can be found in iron III oxide Fe₂O₃. Consider the process of melting the ice for which we write the following chemical equation. There is a standard way of writing chemical equations.
Plus signs are placed between multiple reactants or products. Krebs cycle Click Here for Sample Questions Krebs cycle or tricarboxylic acid cycle TCA cycle or citric acid cycle is a series of chemical reactions of central importance in all living cells that utilises oxygen as part of cellular respiration. The numbers appearing as subscripts in the.
However within the realm of the thermodynamics we must write the chemical equations with change in heat enthalpy change. Where are the reactants and the products located in a chemical equation. Since Fe2O3 and C are on left side of.
The substance s to the right of the arrow are called products. The reactants are on the left side of the arrow and the products are on the right side of the arrowReactants --- Products. Usually on the right of the yeild signOn the right side of the arrow in the equation.
A substances formed from a reaction. In the equation above the zinc and sulfur are the reactants that chemically combine to form the zinc sulfide product. On the night wide of the arrow in the equation c.
A chemical equation is made more informative by mentioning the physical state of the reactants and the products along with their chemical formulae. In chemical equations reactants and products are represented by chemical symbols and formulas. The gaseous liquid aqueous and solid states are abbreviated differently as g l aq and s respectively.
They are all the elements and compounds located on the RIGHT side of chemical equation. The arrow between the reactants and products should point from left to right or if the reaction is proceeding both. A True B False Easy Solution Verified by Toppr Correct option is B It is the convention to place the reactants on the left side of a chemical equation and the products on the right side.
Where are the products in a chemical equation located a on the left side of the snow in the equation. H2O s Æ H2O l 1. In a chemical equation the reactants appears to be on the left.
We will first take the maximum number of atoms present on either side of the reaction that we can find on product side. So in our case the reactants are 2Al s Fe2O3 s. In a chemical equation the products are written on the left-hand side and the reactants on the right-hand side with an arrow in between.
An arrow is used to indicate the direction in which the reaction occurs. The starting materials in a chemical reaction. Reactants Products Yields Coefficients Subscripts.
Thank you for. Then we multiply the number of oxygen atoms on reactant side by 4 such that the number of oxygen atoms on both sides of the reaction is balanced. The chemical equation for this reaction is written as.
You learned that the reactants are always on the left side of a chemical equation and that the products are always on the right side of.

How To Balance Chemical Equations Chemical Equation Equations Chemical

Chemical Reactions Poster Teaching Chemistry Chemical Reactions Matter Science

How To Balance Chemical Equations Chemical Equation Equations Chemistry Lessons

Decomposition Reactions Youtube Chemical Reactions Chemical Equation Reactions

Chemistry11mrstandring Chemical Equations And The Conservation Laws Chemical Equation Chemistry Chemical

Coefficient Easy Science Flashcards Chemical Equation 8th Grade Science

Balancing Chemical Equations Activity Lab Chemical Equation Equations Lab Activities

Balancing Chemical Equations Lesson Plan A Complete Science Lesson Using The 5e Method Of Instructi Chemical Equation Equations Balancing Equations Chemistry
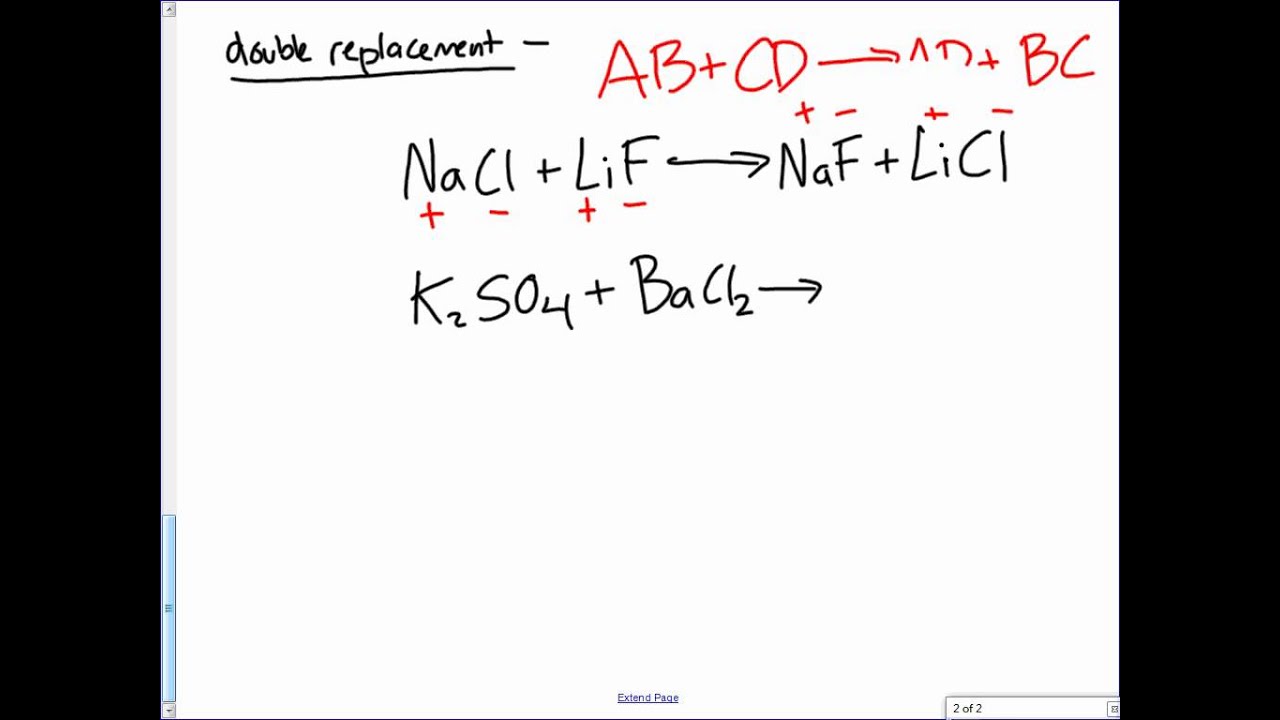
Good Video For Predicting Products Of Various Types Of Chemical Reactions Nicole Galante Chemical Reactions Reactions Cool Gifs

Balancing Chemical Equations Worksheet Customizable Teaching Chemistry Chemistry Lessons Chemistry Education
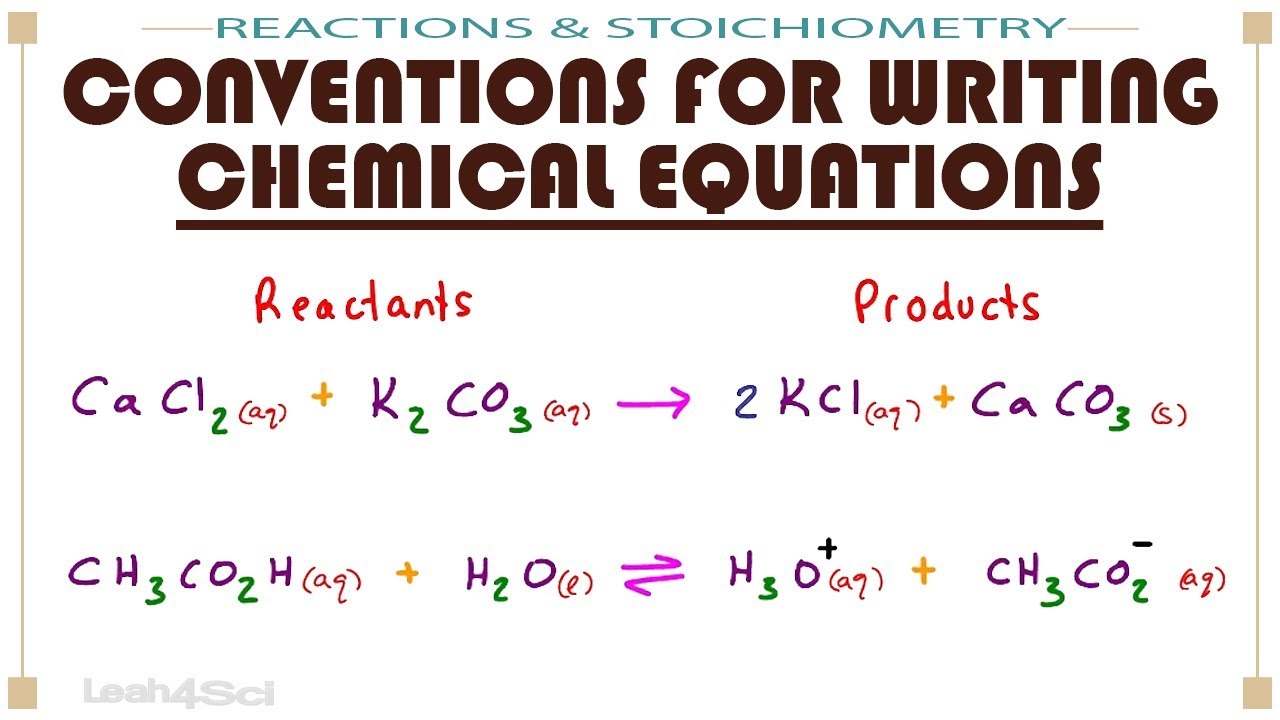
Conventions For Writing Chemical Equations Including Arrows Phases Coefficients Reactants Products And Chemical Equation Chemistry Lessons Chemistry Basics

How To Balance Chemical Equations Chemical Equation Balancing Equations Equations

Classifying Chemical Equations Worksheets Chemical Reactions Reaction Types Redox Reactions

Balanced Chemical Equations Worksheet Chemical Equation Equations Chemistry Worksheets

Types Of Reactions Classwork Homework By Threefourthsme Tpt Chemical Reactions Covalent Bonding Chemical Equation

Predicting The Products Of Chemical Reactions Chemistry Examples And P Chemistry Worksheets Apologia Physical Science Chemistry Lessons

Free Chemical Equations Posters Chemical Equation Physical Science High School Homeschool Science

Easy Steps To Balance Chemical Equations Chemical Equation High School Science Experiments Chemistry Basics

Easy Steps To Balance Chemical Equations Chemical Equation Teaching Chemistry Math Methods
Comments
Post a Comment